ANAND CLASSES Study Material and Notes to learn what a chemical reaction is, how it works, with a detailed example of magnesium and oxygen reaction, and explore real-life chemical changes happening around us every day for Class 10 Science with detailed Q&A, assertion-reason based MCQs, and explanations. Perfect for CBSE, NEET, JEE and school exam preparation.
🧪 What is a Chemical Reaction?
- A chemical reaction is a process in which new substances with entirely new properties are formed.
- It involves chemical changes, where a rearrangement of atoms occurs between the reacting substances.
- During a chemical reaction:
- Old chemical bonds between atoms are broken.
- New chemical bonds are formed between rearranged atoms.
- Importantly, atoms of one element do not change into another. Only their arrangement changes.
🔬 Reactants and Products
- Reactants: Substances that participate in a chemical reaction.
- Products: New substances formed as a result of the chemical reaction.
- The properties of the products are usually completely different from those of the reactants.
For example a general chemical reaction :
A + B → C + D
Here, A and B are the reactants, which react to form the products C and D. In an actual chemical equation, reactants and products are denoted by their chemical formula
🔥 Reaction of Magnesium with Oxygen
Let’s look at a simple yet classic chemical reaction.
🧵 Materials Involved:
- Magnesium (Mg) – Silvery-white metal, usually in ribbon form.
- Oxygen (O2) – Present in air.
🔥 Reaction Process:
When magnesium ribbon is heated:
- It burns in air with a dazzling white flame.
- It forms a white powder called magnesium oxide (MgO).
Chemical Equation: $$\text{Magnesium} + \text{Oxygen} \xrightarrow{\text{Heat}} \text{Magnesium Oxide}$$
(Mg) + (O2) → 2 MgO
- This is a chemical change as the product (MgO) has properties different from the reactants (Mg and O2).

🧽 Why Clean Magnesium Ribbon?
- Magnesium ribbon forms a layer of magnesium oxide over time due to slow reaction with air.
- This oxide coating prevents proper reaction.
- So, the ribbon must be cleaned with sandpaper before burning.
👁 Safety Precautions
- The bright white flame of burning magnesium is harmful to the eyes.
- Always keep the burning ribbon away from direct eyesight.
🧪 Activity: Burning Magnesium Ribbon
🔧 Materials Needed:
- 2 cm long magnesium ribbon
- Sandpaper
- Pair of tongs
- Bunsen burner
- Watch glass
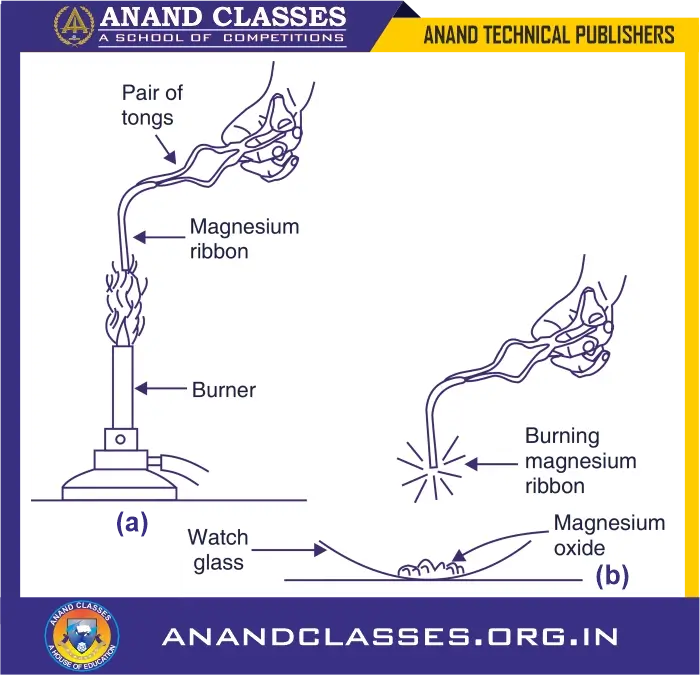
📝 Steps of activity:
- Take about 2 cm long magnesium ribbon and clean it by rubbing its surface with sand paper.
- Hold the magnesium ribbon with a pair of tongs at one end, and heat its other end over a burner
- The magnesium ribbon starts burning with a dazzling white flame.
- Hold the burning magnesium ribbon over a watch glass so that the magnesium oxide powder being formed collects in the watch glass
🌍 Chemical Reactions in Daily Life
Chemical reactions are not just limited to the laboratory. Many everyday processes involve them:
- 🥛 Souring of milk
- 🧫 Formation of curd
- 🍲 Cooking of food
- 🍽 Digestion of food
- 💨 Respiration
- 🍇 Fermentation of grapes
- 🔩 Rusting of iron
- 🔥 Burning of fuels (wood, coal, kerosene, etc.)
- 🕯 Burning of candle wax
- 🍌 Ripening of fruits
In all these changes, the original substance transforms into a new substance with new properties due to a chemical reaction.
Chemical Reaction – Exam Oriented Questions and Answers (Class 10 Science : Chemistry)
Q1: What is a chemical reaction?
A: A chemical reaction is a process in which new substances with new properties are formed. These involve chemical changes and rearrangement of atoms in the reacting substances.
Q2: What happens to atoms during a chemical reaction?
A: During a chemical reaction, atoms of the reactants are rearranged to form new substances. The atoms themselves do not change into atoms of another element.
Q3: What changes occur to chemical bonds in a chemical reaction?
A: Chemical reactions involve the breaking of old chemical bonds between atoms in the reactants and the formation of new chemical bonds in the products.
Q4: What are reactants in a chemical reaction?
A: Reactants are the substances that take part in a chemical reaction.
Q5: What are products in a chemical reaction?
A: Products are the new substances that are formed as a result of a chemical reaction.
Q6: How do the properties of products compare to those of reactants?
A: The products have properties that are entirely different from the properties of the reactants.
Q7: Give an example of a simple chemical reaction.
A: When magnesium ribbon is heated, it burns in air with a dazzling white flame to form a white powder called magnesium oxide.
Chemical Equation:
Magnesium + Oxygen → Magnesium oxide
(Magnesium ribbon + Oxygen from air → White powder)
Q8: What are the reactants and product in the magnesium burning reaction?
A:
- Reactants: Magnesium and Oxygen
- Product: Magnesium Oxide
Q9: Why is magnesium ribbon cleaned before burning?
A: Magnesium ribbon is cleaned with sandpaper to remove the layer of magnesium oxide that forms due to slow reaction with air. This allows fresh magnesium to react readily with oxygen during the experiment.
Q10: Why should magnesium ribbon be kept away from eyes while burning?
A: The dazzling white light produced during the burning of magnesium is very bright and can harm the eyes.
Q11: What are the steps to perform the magnesium burning experiment?
A:
- Take a 2 cm long magnesium ribbon and clean it with sandpaper.
- Hold it with tongs and heat one end over a burner.
- It starts burning with a dazzling white flame.
- Hold it over a watch glass to collect the white magnesium oxide powder formed.
Q12: Can chemical reactions happen outside the laboratory?
A: Yes, many chemical reactions occur in everyday life.
Q13: List some examples of chemical reactions in daily life.
A:
- Souring of milk
- Formation of curd
- Cooking and digestion of food
- Respiration
- Fermentation of grapes
- Rusting of iron
- Burning of fuels (wood, coal, kerosene, LPG)
- Burning of candle wax
- Ripening of fruits
Q14: What common feature is observed in all these daily life chemical reactions?
A: In all these cases, the original substance changes its nature and identity due to the chemical reaction involved.
🧪 Multiple Choice Questions (MCQs) – Chemical Reactions (Class 10 Science)
MCQs (Multiple Choice Questions) based on the topic Chemical Reactions — ideal for CBSE Class 10 Science
Q1. What happens during a chemical reaction?
A. Atoms of elements are destroyed
B. New atoms are created
C. Atoms rearrange to form new substances
D. Molecules become atoms
✅ Correct Answer: C. Atoms rearrange to form new substances
📝 Explanation: In a chemical reaction, atoms are neither created nor destroyed, only rearranged.
Q2. The substances which take part in a chemical reaction are called:
A. Catalysts
B. Reactants
C. Products
D. Elements
✅ Correct Answer: B. Reactants
📝 Explanation: Reactants are the initial substances that undergo change in a chemical reaction.
Q3. What is formed when magnesium burns in air?
A. Magnesium carbonate
B. Magnesium sulphate
C. Magnesium oxide
D. Magnesium hydroxide
✅ Correct Answer: C. Magnesium oxide
📝 Explanation: Magnesium reacts with oxygen to form magnesium oxide (MgO).
Q4. Why is magnesium ribbon cleaned before burning?
A. To make it shine
B. To remove dust
C. To remove oxide layer
D. To break it into pieces
✅ Correct Answer: C. To remove oxide layer
📝 Explanation: Magnesium develops a coating of magnesium oxide on its surface which is removed to ensure proper reaction.
Q5. Which of the following is NOT a chemical change?
A. Rusting of iron
B. Melting of ice
C. Digestion of food
D. Burning of paper
✅ Correct Answer: B. Melting of ice
📝 Explanation: Melting of ice is a physical change, not a chemical reaction.
🔍 Assertion-Reason Based Questions – Chemical Reactions (Class 10 Science)
Q1.
Assertion (A): Magnesium ribbon is cleaned before burning in air.
Reason (R): Cleaning removes the protective layer of magnesium oxide from the ribbon.
Options:
A. Both A and R are true, and R is the correct explanation of A. ✅
B. Both A and R are true, but R is not the correct explanation of A.
C. A is true, but R is false.
D. A is false, but R is true.
Explanation: Magnesium develops a coating of magnesium oxide which prevents proper burning, hence it is removed.
Q2.
Assertion (A): A chemical reaction involves only a physical change.
Reason (R): No new substance is formed during a chemical reaction.
Options:
A. Both A and R are true, and R is the correct explanation of A.
B. Both A and R are true, but R is not the correct explanation of A.
C. A is true, but R is false.
D. A is false, and R is also false. ✅
Explanation: A chemical reaction is a chemical change where new substances are formed.
Q3.
Assertion (A): Chemical reactions involve breaking and making of bonds.
Reason (R): Atoms rearrange during chemical reactions to form new compounds.
Options:
A. Both A and R are true, and R is the correct explanation of A. ✅
B. Both A and R are true, but R is not the correct explanation of A.
C. A is true, but R is false.
D. A is false, but R is true.
Explanation: The rearrangement of atoms results in breaking old bonds and forming new ones.
Q4.
Assertion (A): Burning of magnesium ribbon in air is a chemical change.
Reason (R): A white powdery substance is formed which has different properties from the original.
Options:
A. Both A and R are true, and R is the correct explanation of A. ✅
B. Both A and R are true, but R is not the correct explanation of A.
C. A is true, but R is false.
D. A is false, but R is true.
Explanation: Magnesium reacts with oxygen to form magnesium oxide with new properties, indicating a chemical change.
Q5.
Assertion (A): In a chemical reaction, atoms of elements are converted into new atoms.
Reason (R): Chemical reactions involve formation of new elements.
Options:
A. Both A and R are true, and R is the correct explanation of A.
B. Both A and R are true, but R is not the correct explanation of A.
C. A is true, but R is false.
D. A is false, and R is also false. ✅
Explanation: Atoms are not converted into new atoms; they are simply rearranged.
🧪 Case Study – Chemical Reactions (Class 10 Science)
This Case Study helps students understand the real-world application of chemical reactions, and the questions test their ability to identify key concepts like the formation of new substances, the nature of chemical reactions, and the role of reactants and products.
Case Study: Burning of Magnesium Ribbon
In a science laboratory, a magnesium ribbon is taken and cleaned by rubbing it with sandpaper to remove the protective layer of magnesium oxide that forms on its surface when exposed to air. The cleaned ribbon is then heated using a burner. As the ribbon heats up, it begins to burn with a dazzling white flame, producing a white powdery substance. This substance is called magnesium oxide (MgO). The reaction occurs in the presence of oxygen from the air.
The reaction can be represented as:
Magnesium + Oxygen → Magnesium Oxide
The magnesium oxide formed is a white powder, and it has very different properties compared to the original magnesium ribbon and oxygen.
Magnesium oxide is used in various applications, including in fire-resistant materials and as a component in certain chemical reactions.
Questions:
Q1. What is the purpose of cleaning the magnesium ribbon before burning it in air?
A. To make the ribbon shiny
B. To remove the protective layer of magnesium oxide
C. To increase the temperature of the ribbon
D. To reduce the weight of the ribbon
Q2. What is the chemical equation for the burning of magnesium in air?
A. 2Mg + O₂ → 2MgO
B. Mg + O₂ → MgO
C. Mg + H₂O → Mg(OH)₂
D. MgO + O₂ → MgO
Q3. Which of the following statements is true about the product formed during the burning of magnesium?
A. Magnesium oxide has the same properties as magnesium
B. Magnesium oxide is a gas
C. Magnesium oxide is a white powder with different properties than magnesium
D. Magnesium oxide is liquid at room temperature
Q4. In the reaction of magnesium and oxygen, what type of change occurs?
A. Physical change
B. Chemical change
C. Both physical and chemical change
D. No change occurs
Q5. Why is the burning of magnesium ribbon considered a chemical reaction?
A. New substances with different properties are formed
B. No new substance is formed
C. The magnesium ribbon melts during the process
D. The magnesium ribbon changes color
Answer Key:
- B. To remove the protective layer of magnesium oxide
- B. Mg + O₂ → MgO
- C. Magnesium oxide is a white powder with different properties than magnesium
- B. Chemical change
- A. New substances with different properties are formed
🧪 Worksheet – Chemical Reactions (Class 10)
This worksheet covers multiple-choice questions, assertion-reason questions, case studies, and both short and long answer questions to assess students’ understanding of Chemical Reactions.
Name: ____________________
Date: ____________________
Class: 10
Section A: Multiple Choice Questions (MCQs)
Q1. What is the product formed when magnesium burns in air?
A. Magnesium carbonate
B. Magnesium oxide
C. Magnesium sulfate
D. Magnesium hydroxide
Q2. Which of the following is a physical change?
A. Burning of wood
B. Melting of ice
C. Rusting of iron
D. Cooking of food
Q3. In a chemical reaction, the substances that undergo change are called:
A. Products
B. Catalysts
C. Reactants
D. New substances
Q4. The burning of a candle is an example of a:
A. Physical change
B. Chemical change
C. Nuclear reaction
D. Both physical and chemical change
Q5. Which of the following is NOT a chemical change?
A. Rusting of iron
B. Burning of fuel
C. Dissolving salt in water
D. Cooking of food
Section B: Assertion-Reason Questions
Q6.
Assertion (A): During a chemical reaction, new substances are formed.
Reason (R): Chemical reactions involve the rearrangement of atoms.
Options:
A. Both A and R are true, and R is the correct explanation of A.
B. Both A and R are true, but R is not the correct explanation of A.
C. A is true, but R is false.
D. A is false, and R is true.
Q7.
Assertion (A): Magnesium ribbon is cleaned before burning.
Reason (R): The protective layer of magnesium oxide prevents magnesium from burning.
Options:
A. Both A and R are true, and R is the correct explanation of A.
B. Both A and R are true, but R is not the correct explanation of A.
C. A is true, but R is false.
D. A is false, but R is true.
Section C: Case Study
Case Study:
In a laboratory, a magnesium ribbon is taken, cleaned by rubbing it with sandpaper, and heated using a burner. It burns with a dazzling white flame, producing a white powdery substance known as magnesium oxide (MgO).
Q8. Why is the magnesium ribbon cleaned before burning it?
Q9. What is the white powder formed during the burning of magnesium called?
Q10. What is the nature of the chemical change during this process?
Q11. Write the chemical equation for the burning of magnesium in oxygen.
Section D: Short Answer Questions
Q12. Define a chemical reaction and explain with an example.
Q13. How can you identify a chemical change has occurred? List at least three indicators.
Q14. Explain the difference between reactants and products in a chemical reaction.
Q15. Describe the process of rusting and explain why it is a chemical change.
Section E: Long Answer Questions
Q16. Explain the process of burning magnesium ribbon in air. Describe the reaction and the nature of the product formed.
Q17. Describe the difference between a physical change and a chemical change, with examples.
Answer Key
MCQs:
- B. Magnesium oxide
- B. Melting of ice
- C. Reactants
- B. Chemical change
- C. Dissolving salt in water
Assertion-Reason: 6. A. Both A and R are true, and R is the correct explanation of A.
7. A. Both A and R are true, and R is the correct explanation of A.
🔍 Do You Know?
“Do You Know?” and Quick Revision Points for Class 10 Science – Chemical Reactions, designed to help students grasp the key concepts in a concise and engaging way.
- Magnesium Ribbon:
Magnesium ribbon has a layer of magnesium oxide on its surface when exposed to air. This layer prevents it from burning properly, which is why it is cleaned before burning. - Chemical Reactions in Daily Life:
Many chemical reactions happen around us every day, such as rusting of iron, ripening of fruits, and digestion of food. These reactions involve changes in the properties of the substances. - Dazzling Light:
When magnesium burns, it gives off a dazzling white light. This intense light can be harmful to the eyes, so it’s important to view it from a safe distance or use protective glasses. - Exothermic Reactions:
Some chemical reactions, like the burning of magnesium, release energy in the form of heat and light. These are known as exothermic reactions. - Rusting:
Rusting of iron is a chemical change that occurs when iron reacts with oxygen and water, forming iron oxide (rust). This process can be prevented by keeping iron objects dry or by applying a protective coating.
⚡ Quick Revision Points
- Chemical Reactions involve the transformation of reactants into products with new properties. This process includes the breaking and making of chemical bonds.
- Reactants are the substances that undergo a chemical change, and products are the new substances formed as a result of the reaction.
- Magnesium Oxide Formation:
When magnesium (Mg) reacts with oxygen (O₂) in the air, it forms magnesium oxide (MgO), a white powder.
The balanced chemical equation for this reaction is:
2Mg + O₂ → 2MgO. - Signs of a Chemical Reaction:
- Change in color
- Release of gas (bubbling or effervescence)
- Formation of a precipitate
- Change in temperature (heat release or absorption)
- Types of Chemical Reactions:
- Combination reaction: Two or more substances combine to form a single product.
- Decomposition reaction: A single compound breaks down into two or more simpler substances.
- Displacement reaction: A more reactive element displaces a less reactive element in a compound.
- Double displacement reaction: Two compounds exchange ions to form two new compounds.
- Exothermic and Endothermic Reactions:
- Exothermic reactions release energy (e.g., combustion of magnesium).
- Endothermic reactions absorb energy (e.g., photosynthesis).
- Examples of Chemical Reactions in daily life:
- Respiration: Glucose reacts with oxygen to release energy.
- Fermentation: Yeast ferments sugar to produce alcohol and carbon dioxide.
- Souring of milk: Lactic acid bacteria convert lactose into lactic acid, causing milk to sour.
- Conservation of Mass:
In a chemical reaction, mass is always conserved. The total mass of the reactants is equal to the total mass of the products.








