ANAND CLASSES Study Material and Notes to explore the key characteristics of chemical reactions such as gas evolution, precipitate formation, color change, temperature change, and change in state with simple examples and explanations. Ideal for CBSE Class 10 students and JEE, NEET Aspirants.
Characteristics of Chemical Reactions
In a chemical reaction, substances called reactants are transformed into new substances known as products. During this transformation, we often observe visible changes. These observable features are known as the characteristics of chemical reactions.
🔬 Key Characteristics of Chemical Reactions
The main characteristics that indicate a chemical reaction has occurred include:
- 🔥 Evolution of a gas
- 🌫️ Formation of a precipitate
- 🎨 Change in colour
- 🌡️ Change in temperature
- 💧 Change in state
Each of these characteristics is explained below with easy-to-understand examples.
🔥 1. Evolution of a Gas
Some reactions produce gas bubbles, indicating the formation of a new gaseous product.
🧪 Example.1 :
When zinc granules react with dilute sulphuric acid, then bubbles of hydrogen gas are produced around zinc granules. So, the chemical reaction between zinc and dilute sulphuric acid is characterised by the evolution of hydrogen gas.
Zinc + Dilute Sulphuric Acid → Hydrogen Gas
Observation : Bubbles of hydrogen gas form around zinc granules.
Additional Note: Flask becomes warm due to heat release.
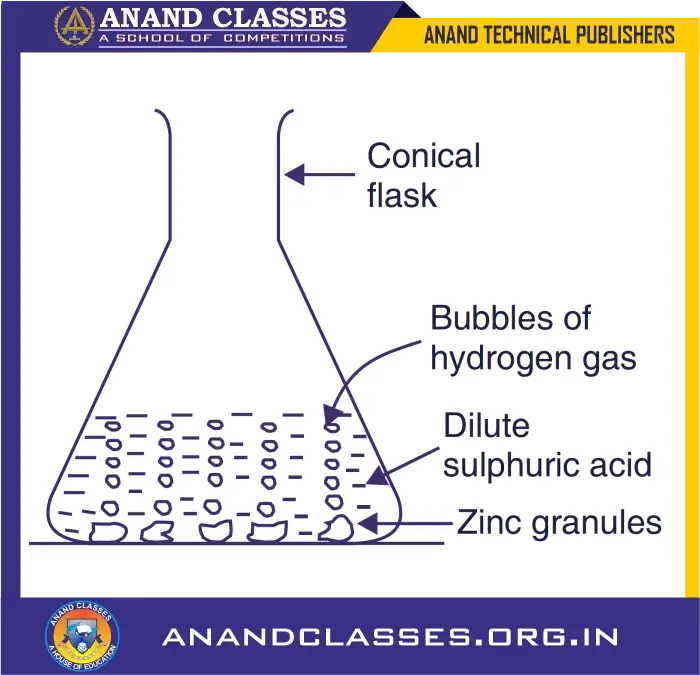
We can perform this chemical reaction in the laboratory as follows :
- Take some zinc granules in a conical flask (or a test-tube).
- Add dilute sulphuric acid over zinc granules.
- We will see the bubbles of hydrogen gas being formed around zinc granules (see Figure).
- If we touch the conical flask with our hand, we will find that it is somewhat hot. So, a change in temperature (rise in temperature) also occurs in this chemical reaction.
🧪 Balanced Chemical Equation:
$$\text{Zn} + \text{H}_2\text{SO}_4 \rightarrow \text{ZnSO}_4 + \text{H}_2 \uparrow$$
🧪 Explanation:
- When zinc (Zn) reacts with dilute sulphuric acid (H2SO4), it displaces hydrogen from the acid.
- The reaction produces zinc sulphate (ZnSO4) and hydrogen gas (H2).
- This reaction is an example of a single displacement reaction, where zinc (Zn) displaces hydrogen (H2) from dilute sulphuric acid (H2SO4).
- Bubbles of hydrogen gas can be observed during the reaction.
- Heat is also released, and the reaction is exothermic.
💡 Do You Know? Hydrogen gas produced in this reaction can be tested by bringing a burning matchstick near it — it burns with a ‘pop’ sound, confirming the presence of hydrogen!
🧪 Example.2 :
When magnesium reacts with a dilute acid (like dilute hydrochloric
acid or dilute sulphuric acid), even then hydrogen gas is evolved (see
Figure).
Magnesium + Dilute Hydrochloric Acid → Hydrogen Gas

🧪 Balanced Chemical Equation:
$$\text{Mg} + 2\text{HCl} \rightarrow \text{MgCl}_2 + \text{H}_2 \uparrow$$
🧪 Explanation:
- When magnesium (Mg) reacts with dilute hydrochloric acid (HCl), magnesium chloride (MgCl2) and hydrogen gas (H2) are produced.
- This reaction is an example of a single displacement reaction, where magnesium displaces hydrogen from hydrochloric acid.
- Bubbles of hydrogen gas evolve during the reaction, demonstrating the evolution of gas, one of the key characteristics of chemical reactions.
- Heat is also released, and the reaction is exothermic.
💡 Do You Know? The hydrogen gas released during this reaction can be tested using the ‘pop’ test. When a burning matchstick is brought near the gas, it makes a distinct popping sound, confirming the presence of hydrogen.
🧪 Example.3 :
The chemical reaction between sodium carbonate and dilute hydrochloric acid is characterised by the evolution of carbon dioxide gas.
Sodium Carbonate + Dilute Hydrochloric Acid → Carbon Dioxide
Observation: Effervescence due to CO2 gas.
🧪 Balanced Chemical Equation:
$$\text{Na}_2\text{CO}_3 + 2\text{HCl} \rightarrow 2\text{NaCl} + \text{H}_2\text{O} + \text{CO}_2 \uparrow$$
🧪 Explanation:
- When sodium carbonate (Na2CO3) reacts with dilute hydrochloric acid (HCl), sodium chloride (NaCl), water (H2O), and carbon dioxide (CO2) are produced.
- Carbon dioxide gas is evolved as one of the products, which is a clear indicator of a chemical reaction taking place.
- The gas can be captured and tested by bubbling it through limewater (a solution of calcium hydroxide), which turns milky due to the formation of calcium carbonate.
🔬 Test for Carbon Dioxide (CO2)
When carbon dioxide (CO2) gas is passed through limewater (a solution of calcium hydroxide, Ca(OH)2), the limewater turns milky due to the formation of calcium carbonate (CaCO3). This is a simple and common test to confirm the presence of carbon dioxide gas.
Chemical Reaction:
$$\text{CO}_2 + \text{Ca(OH)}_2 \rightarrow \text{CaCO}_3 \, (\text{white precipitate}) + \text{H}_2\text{O}$$
🧪 Explanation:
- The calcium carbonate (CaCO3) produced is a white precipitate that makes the limewater turn milky.
- This reaction is often used in laboratory experiments to confirm the presence of carbon dioxide gas.
💡 Do You Know? If you continue to pass carbon dioxide gas through the milky limewater, the milkiness will eventually disappear due to the formation of calcium bicarbonate:
$$\text{CaCO}_3 + \text{CO}_2 + \text{H}_2\text{O} \rightarrow \text{Ca(HCO₃)₂}$$
This reversible reaction shows that excess CO2 dissolves in limewater.
💡 Do You Know? The carbon dioxide produced can also be tested using a burning splint. When passed through carbon dioxide, a glowing splint will be extinguished, confirming the presence of CO2.
🌫️ 2. Formation of a Precipitate
A precipitate is a ‘solid product’ which separates out from the solution during a chemical reaction.
A precipitate can be formed by mixing aqueous solutions (water solutions) of reactants when one of the products is insoluble in water.
A precipitate can also be formed by passing a gas into an aqueous solution of a substance (like passing carbon dioxide gas into lime water).
🧪 Example.1 :
When potassium iodide solution is added to a solution of lead nitrate, then a yellow precipitate of lead iodide is formed.
Lead Nitrate + Potassium Iodide → Yellow Precipitate (Lead Iodide)
Observation: Instant yellow solid appears in the solution.
Also: Colour change from colourless to yellow.

🧪 Balanced Chemical Equation:
$$\text{Pb(NO}_3)_2 (aq) + 2\text{KI} (aq) \rightarrow \text{PbI}_2 (s) \downarrow + 2\text{KNO}_3 (aq)$$
🧬 Explanation:
- When an aqueous solution of lead nitrate (Pb(NO3)2) is mixed with an aqueous solution of potassium iodide (KI), a yellow precipitate of lead iodide (PbI2) is formed.
- The yellow solid (PbI2) is insoluble in water, which causes it to settle at the bottom of the test tube.
- This reaction is a classic example of a precipitation reaction, where two soluble salts react to form an insoluble salt (precipitate).
📌 Observation:
- Formation of a bright yellow precipitate immediately upon mixing the two colorless solutions.
💡 Do You Know? The yellow precipitate of lead iodide is more soluble in hot water and recrystallizes into shiny golden crystals upon cooling, earning it the nickname “Golden Rain” in chemistry demonstrations.
🧪 Example.2 :
When dilute sulphuric acid is added to barium chloride solution taken in a test-tube, then a white precipitate of barium sulphate is formed
Barium Chloride + Dilute Sulphuric Acid → White Precipitate (Barium Sulphate)
Observation: Cloudy white substance forms.
🧪 Balanced Chemical Equation:
$$\text{BaCl}_2 (aq) + \text{H}_2\text{SO}_4 (aq) \rightarrow \text{BaSO}_4 (s) \downarrow + 2\text{HCl} (aq)$$
🧬 Explanation:
- When barium chloride (BaCl2) solution is added to dilute sulphuric acid (H2SO4), a white precipitate of barium sulphate (BaSO4) is formed.
- This white solid is insoluble in water and appears as a milky suspension, settling down as a precipitate.
- This is an example of a precipitation reaction, indicating the formation of an insoluble salt.
📌 Observation:
- Immediate appearance of a dense white precipitate when the two clear solutions are mixed.
💡 Do You Know? Barium sulphate is so insoluble that it’s used in medical imaging (barium meals) to outline the stomach and intestines in X-ray diagnosis — it’s safe because it doesn’t dissolve in the body.
🎨 3. Change in Colour
Some reactions cause a visible change in colour, signaling the formation of new substances.
🧪 Example.1 :
When citric acid reacts with potassium permanganate solution, then the purple colour of potassium permanganate solution disappears (it becomes colourless).
Citric Acid (Lemon Juice) + Potassium Permanganate (Purple) → Colourless
Observation : Purple colour gradually fades to colourless.
🧪 Balanced Chemical Equation :
$$\text{C}_6\text{H}_8\text{O}_7 + \text{KMnO}_4 \rightarrow \text{Mn}^{2+} + \text{CO}_2 + \text{H}_2\text{O}$$
🧬 Explanation:
- Citric acid (present in lemon juice) is a reducing agent, and potassium permanganate (KMnO4) is a strong oxidizing agent.
- When citric acid is added dropwise to a purple KMnO4 solution, the purple colour fades and eventually disappears, making the solution colourless.
- This is due to the reduction of Mn7+ ions (purple) to Mn2+ ions (colourless).
- This reaction is an example of a redox reaction and a change in colour, which is a characteristic of chemical reactions.
📌 Observation:
- Gradual disappearance of the purple colour of potassium permanganate as lemon juice is added.
💡 Do You Know? Potassium permanganate is used as a disinfectant and water purifier. Its intense colour makes it perfect for visually observing redox changes!
🧪 Example.2 :
When sulphur dioxide gas is passed through acidified potassium dichromate solution, then the orange colour of potassium dichromate solution changes to green.
Sulphur Dioxide + Acidified Potassium Dichromate (Orange) → Green
Observation : Orange solution turns green.

🧪 Balanced Chemical Equation :
$$\text{SO}_2 + \text{K}_2\text{Cr}_2\text{O}_7 + \text{H}_2\text{SO}_4 \rightarrow \text{Cr}^{3+} (\text{green}) + \text{K}_2\text{SO}_4 + \text{H}_2\text{O}$$
🧬 Explanation:
- Sulphur dioxide (SO2) is a reducing agent, and acidified potassium dichromate (K2Cr2O7 + H2SO4) is an oxidizing agent.
- When SO2 is passed through acidified potassium dichromate, the orange colour of dichromate ions (Cr2O72-) is reduced to green Cr3+ ions.
- This is a classic example of a redox reaction and a characteristic colour change in chemical reactions.
📌 Observation:
- The solution changes colour from orange to green, indicating that a chemical reaction has occurred.
💡 Do You Know? This reaction is often used as a chemical test for the presence of SO2 gas and is an important demonstration of oxidation-reduction reactions in the lab.
🌡️ 4. Change in Temperature
Chemical reactions often release or absorb heat, resulting in a temperature change.
When a chemical reaction produces heat energy (exothermic reactions), then the temperature of reaction mixture rises (or increases) and it becomes hot.
When a chemical reaction absorbs heat energy (endothermic reactions), then the temperature of reaction mixture falls (or decreases) and it becomes cold.
So, when we talk of ‘change in temperature’ in a chemical reaction, it can be ‘rise in temperature’ or ‘fall in temperature
🧪 Example.1 :
When quicklime reacts with water, then slaked lime is formed and a lot of heat energy is produced. This heat raises the temperature due to which the reaction mixture becomes hot.
Quicklime (CaO) + Water → Slaked Lime (Ca(OH)2) + Heat
Observation: Beaker becomes hot (exothermic reaction).
We can perform this chemical reaction as follows :
- (i) Take a little of quicklime in a hard-glass beaker [See Figure].

- (ii) Add water to it slowly [See Figure].
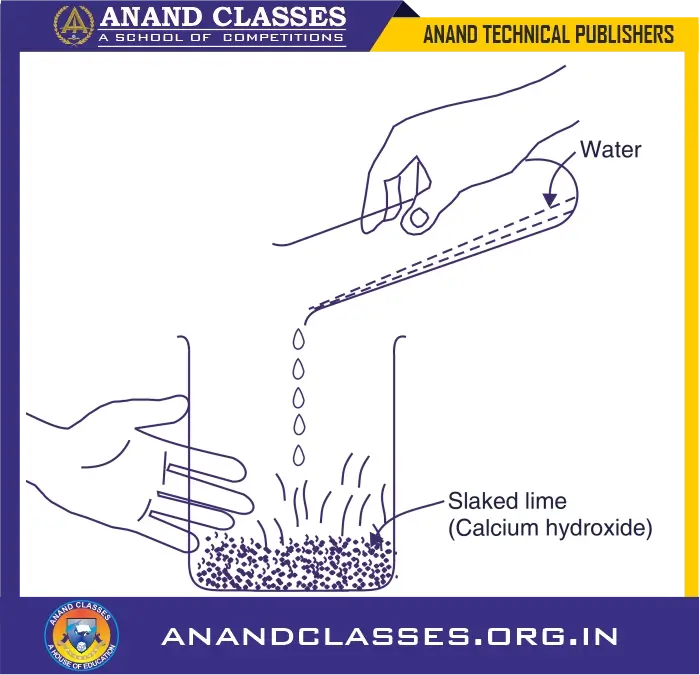
The beaker becomes hot. Its temperature rises.
- (iii) Touch the beaker carefully.
- (iv) The beaker feels to be quite hot (Its temperature is high).
🧪 Balanced Chemical Equation:
$$\text{CaO (s)} + \text{H}_2\text{O (l)} \rightarrow \text{Ca(OH)}_2 \text{(aq)} + \text{Heat}$$
🧪 Explanation:
- Quicklime (Calcium Oxide, CaO) reacts vigorously with water (H2O) to form slaked lime (Calcium Hydroxide, Ca(OH)2).
- This reaction releases a lot of heat, making it an exothermic reaction.
- The temperature of the container rises, and the beaker becomes hot to touch.
📌 Observation:
- A hissing sound may be heard.
- The beaker gets hot, and a milky suspension of slaked lime is formed.
🔥 Type of Reaction:
- Combination Reaction
- Exothermic Reaction
- Change in Temperature (Rise)
💡 Do You Know? Slaked lime is used in whitewashing walls, neutralizing acidic soils, and in water treatment processes.
🧪 Example.2 :
Chemical reaction between zinc granules and dilute sulphuric acid to produce hydrogen gas. If we touch the conical flask containing zinc granules and dilute sulphuric acid, it is found to be warm (which means that the temperature rises during this reaction).
Zinc + Dilute Sulphuric Acid → Hydrogen + Heat
Observation: Flask gets warm.
🧪 Balanced Chemical Equation:
$$\text{Zn} + \text{H}_2\text{SO}_4 \rightarrow \text{ZnSO}_4 + \text{H}_2 \uparrow$$
🧪 Example.3 :
When barium hydroxide (Ba(OH)2) is added to ammonium chloride (NH4Cl) taken in a test-tube and mixed with a glass rod, then barium chloride, ammonia and water are formed. A lot of heat energy is absorbed during this reaction due to which the temperature of reaction mixture falls and the bottom of test-tube becomes very cold.
Barium Hydroxide + Ammonium Chloride → Barium Chloride + Ammonia + Water
Observation: Test-tube becomes cold (endothermic reaction).
🧪 Balanced Chemical Equation:
$$\text{Ba(OH)}_2 + 2\text{NH}_4\text{Cl} \rightarrow \text{BaCl}_2 + 2\text{NH}_3 + 2\text{H}_2\text{O}$$
🧪 Explanation:
- Barium hydroxide (Ba(OH)2) reacts with ammonium chloride (NH4Cl) in a cold reaction.
- This reaction forms barium chloride (BaCl2), ammonia gas (NH3), and water (H2O).
- It absorbs heat from the surroundings, making the test tube feel very cold.
📌 Observation:
- The bottom of the test tube becomes cold and may even freeze to the table.
- A pungent smell of ammonia is detected.
❄️ Type of Reaction:
- Endothermic Reaction (absorbs heat)
- Double Displacement Reaction
- Change in Temperature (Fall)
💡 Do You Know? This reaction is used in demonstrating endothermic reactions in school labs. The cooling effect is strong enough to freeze water in some conditions.
🧪 Example.4 :
The chemical reaction in which carbon burns in air to form carbon dioxide also releases a lot of heat (see Figure).

🧪 Balanced Chemical Equation:
$$\text{C (Carbon)} + \text{O}_2 \rightarrow \text{CO}_2 + \text{Heat}$$
🧪 Explanation:
- When carbon (in the form of charcoal or graphite) burns in air, it reacts with oxygen (O2).
- This reaction forms carbon dioxide (CO2).
- A large amount of heat is released during this process.
📌 Observation:
- Bright glow or flame is seen while carbon burns.
- The surrounding area becomes hot due to the exothermic nature of the reaction.
🔥 Type of Reaction:
- Exothermic Reaction (releases heat)
- Combustion Reaction
- Change in Temperature (Rise)
- Chemical Change with change in state (solid carbon → gaseous CO2)
💡 Do You Know?
- This is the same principle used in coal-fired power plants, where carbon-rich fuels are burned to generate energy.
- The carbon dioxide produced is a greenhouse gas that contributes to global warming.
💧 5. Change in State
Some reactions result in a change of physical state (solid ↔ liquid ↔ gas).
Example.1 :
🕯️ Combustion of Wax
When wax is burned (in the form of a wax candle), then water and carbon dioxide are formed (see Figure). Now, wax is a solid, water is a liquid whereas carbon dioxide is a gas. This means that during the combustion
reaction of wax, the physical state changes from solid to liquid and gas.

Thus, the combustion reaction of candle wax is characterised by a change in state from solid to liquid and gas (because wax is a solid, water formed by the combustion of wax is a liquid at room temperature whereas carbon dioxide produced by the combustion of wax is a gas).
Wax (Solid) → Carbon Dioxide (Gas) + Water (Liquid)
Observation: Burning candle shows solid wax turning into gas and liquid products.
🔄 Reactions Showing Multiple Characteristics
Some reactions exhibit more than one characteristic at the same time.
📌 Examples:
- Zinc + Dilute Sulphuric Acid → Hydrogen + Heat
- Characteristics: Gas evolution and Temperature rise
- Lead Nitrate + Potassium Iodide → Lead Iodide (Yellow Precipitate)
- Characteristics: Precipitate formation and Colour change
🧠 Do You Know?
- Quicklime is another name for Calcium Oxide (CaO).
- Slaked Lime refers to Calcium Hydroxide (Ca(OH)₂).
- Reactions that release heat are called Exothermic Reactions.
- Reactions that absorb heat are called Endothermic Reactions.
✅ Quick Revision Points
- Chemical reactions can be recognized by changes like gas formation, precipitate, colour, temperature, or state.
- Exothermic reactions feel warm, while Endothermic ones feel cold.
- Precipitates are formed when insoluble solids appear during reactions.
- Colour changes indicate the creation of new substances.