ANAND CLASSES Study Material and Notes to learn the concept “Matter is Made Up of Particles” from Class 9 Science Chapter Physical Nature of Matter with detailed notes, MCQs, assertion-reason questions, and case study-based Q&A. Perfect for CBSE exam preparation.
🔍 Chapter: Matter in Our Surroundings
🧪 Topic – Physical Nature of Matter
Q1. What is meant by “Physical Nature of Matter”?
Ans:
The physical nature of matter deals with how matter exists, what it’s made of, and how it behaves. It explains that matter is made up of tiny particles and that these particles are extremely small, with specific properties like motion, space between them, and forces of attraction.
📌 MATTER IS MADE UP OF PARTICLES
Q2. What is the basic assumption about the nature of matter?
Ans:
The basic assumption is that matter is made up of particles. These particles are so small that they cannot be seen even with a powerful microscope.
Q3. What were the two ancient beliefs about the nature of matter?
Ans:
- Continuous theory: Matter is continuous, like a solid block with no spaces (like a wooden block).
- Particulate theory: Matter is made of very tiny, discrete particles (like grains of sand).
Scientific evidence now supports the particulate theory.
Q4. How can we prove that matter is made up of particles? Explain with an activity.
Ans:
We can perform the following activity:
🔬 Activity-1: Dissolving Sugar/Salt in Water
Steps:
- Take a 100 mL beaker.
- Fill it halfway (about 50 mL) with water.
- Mark the water level.
- Add a spoonful of salt or sugar and stir well.
- Observe the level of water.

Observation:
- The salt or sugar dissolves completely in water.
- There is no rise in the water level.
Conclusion:
- The salt/sugar particles are small enough to fit in the spaces between water particles.
- This indicates that both water and sugar are made of particles, and there is space between these particles.
Q5. What happens to sugar or salt when it dissolves in water? Where does it go?
Ans:
When sugar or salt is added to water:
- Its particles break down into tiny particles.
- These particles spread throughout the water and occupy the empty spaces between water molecules.
- This is why they seem to “disappear,” even though they are still present.
📌 HOW SMALL ARE THESE PARTICLES OF MATTER?
Q6. How can we prove that particles of matter are extremely small?
Ans:
We can prove this with the following experiment:
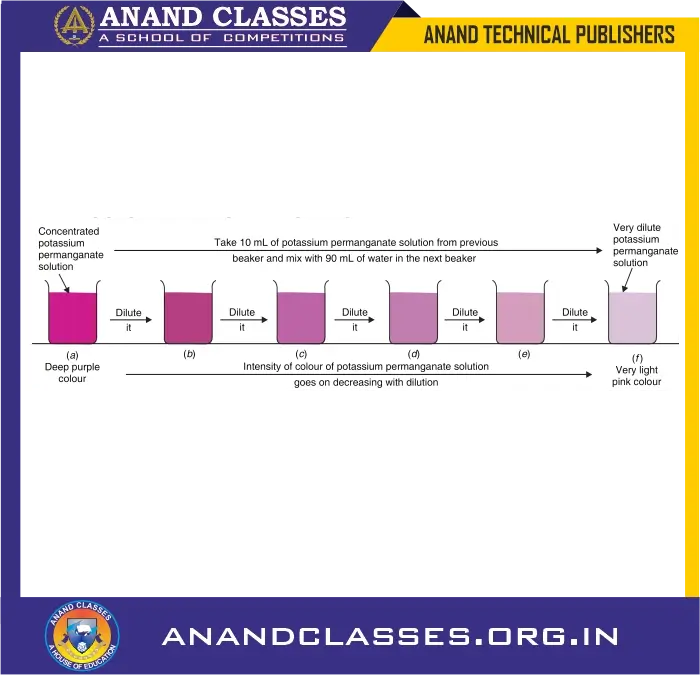
🔬 Activity-2: Dilution of Potassium Permanganate
Steps:
- Take 2–3 crystals of potassium permanganate and dissolve them in 100 mL of water.
- Take 10 mL of this purple solution and add it to another 90 mL of water.
- Again, take 10 mL of this new solution and mix it with another 90 mL of fresh water.
- Repeat this dilution 5 to 8 times.
permanganate persists in the solution
Observation:
- The water remains coloured even after multiple dilutions.
- The intensity of colour reduces, but the purple tint is still visible.
Conclusion:
- Even 2–3 crystals of potassium permanganate contain millions of tiny particles.
- These particles are capable of colouring a very large volume of water.
- Thus, particles of matter are extremely small and can divide further and spread widely.
Q7. Can this experiment be done using other substances?
Ans:
Yes. A similar result can be obtained using Dettol (an antiseptic liquid):
- Add 2 mL of Dettol to water.
- Even after multiple dilutions, the smell of Dettol can still be detected.
- This proves that Dettol’s particles spread in air/water and are extremely small.
Q8. What are the main conclusions from these two activities?
Ans:
- Matter is made up of particles.
- Particles of matter have space between them.
- Particles are extremely small—beyond imagination.
- Particles can mix and spread evenly (diffuse) into another substance.
- Even a small amount of matter contains millions of particles.
Absolutely! Here are detailed explanations for each of the conceptual questions based on the topic “Physical Nature of Matter” (Section 1.1.1 & 1.1.2) – suitable for CBSE Class 9, foundational for JEE/NEET.
🧠 Conceptual Questions with Explanations
Q1. When sugar dissolves in water, the water level does not rise. What does this observation tell us about the nature of matter?
Explanation:
This shows that sugar particles are small and fit into the empty spaces between the water particles. This proves:
- Matter has particles.
- There are spaces (inter-particle spaces) between them.
- Sugar does not occupy extra volume, it fills the gaps.
Q2. If matter were continuous and not made up of particles, what would happen when salt is added to water?
Explanation:
If matter were continuous, there would be no empty spaces between water molecules. So, salt would:
- Not dissolve as there would be no room to go into.
- Increase the water level if forced in. But since salt dissolves without increasing volume, it proves matter is particulate, not continuous.
Q3. You add 2 mL of Dettol to water and shake it. Even after diluting it multiple times, the smell remains. What does this indicate about the particles of Dettol?
Explanation:
It shows that:
- Particles are very small.
- They spread throughout the water and even into the air.
- Even a tiny amount contains millions of particles, enough to create smell in large volume.
Q4. In the potassium permanganate experiment, even after 8 rounds of dilution, the solution is faintly colored. Explain why.
Explanation:
Each time we dilute:
- A small number of permanganate particles remain, yet they are enough to color the solution.
- This shows that each crystal has millions of particles, which keep dividing and spreading even in large volumes.
Q5. Can you see the particles of salt or sugar after they dissolve in water? Why or why not?
Explanation:
No, because:
- The particles break into tiny, invisible particles.
- They uniformly mix with water and occupy inter-particle spaces.
- They become so small and evenly distributed that they are not visible to the naked eye.
Q6. Why do you think a small amount of potassium permanganate can color a large volume of water?
Explanation:
Because:
- Each crystal contains a very large number of particles.
- These particles are extremely small and spread uniformly in water.
- Even a few particles can impart color to a large quantity of water, proving the minute size and effectiveness of particles.
Q7. Suppose you add two substances to water. One increases the water level and the other doesn’t. What can you infer about their particle sizes and nature?
Explanation:
- If the substance increases water level, its particles are too large to fit into the spaces between water particles.
- If the water level doesn’t change, its particles are tiny enough to fit into the inter-particle spaces.
- This shows the importance of particle size in mixing and solubility.
Q8. If we consider that matter is made up of particles, what can you infer about the nature of air, perfume, or gas leaks?
Explanation:
- These substances are gases, and their particles are far apart and move freely.
- Perfume or gas leaks spread in all directions due to the movement of particles — this process is called diffusion.
- The particulate nature and motion of particles explain how they can travel through air.
Q9. How would the particle nature of matter explain why perfumes can be smelled from a distance?
Explanation:
- The tiny particles of perfume evaporate and mix with air particles.
- These particles move in all directions due to random motion and diffuse through air.
- As a result, the smell travels and can be detected even far from the source.
Q10. What property of matter allows a small amount of Dettol or potassium permanganate to affect a large amount of water or air?
Explanation:
- This is due to the very small size of particles and their ability to spread uniformly (diffusion).
- Even a small quantity contains a large number of particles, which can affect large volumes of solvent or air.
- It also shows the uniformity and mobility of particles in a medium.
✅ Bonus Analytical Question:
Q11. A teacher gives you a colorless solution and asks you to test if it has potassium permanganate using dilution steps. What would you do, and what will be your observation if it does?
Explanation:
- Take 10 mL of the given solution and dilute it with 90 mL of water.
- Repeat this 5–8 times.
- If it still shows a purple/pink tinge, it contains potassium permanganate.
- This is because even highly diluted KMnO₄ shows color, proving presence of its particles.
📝 MCQs with Explanations: Physical Nature of Matter
Q1. Which of the following observations supports the idea that matter is made up of particles?
A) Water boils at 100°C
B) Sugar dissolves in water without changing water level
C) Ice melts into water
D) Air expands on heating
✅ Correct Answer: B) Sugar dissolves in water without changing water level
Explanation:
This shows that sugar particles fit into spaces between water particles. It proves the particulate nature of matter and the presence of spaces between particles.
Q2. In the potassium permanganate experiment, a small amount of crystals colors a large volume of water. What does this indicate?
A) Potassium permanganate is a strong chemical
B) Water enhances color
C) Potassium permanganate dissolves completely
D) Particles of matter are very small and spread uniformly
✅ Correct Answer: D) Particles of matter are very small and spread uniformly
Explanation:
Even a tiny amount colors a large volume, proving that the particles are extremely small and capable of diffusing widely in water.
Q3. The smell of perfume spreads across a room because:
A) Perfume is lighter than air
B) Perfume reacts with air
C) Perfume particles move randomly and mix with air
D) Perfume turns into gas immediately
✅ Correct Answer: C) Perfume particles move randomly and mix with air
Explanation:
This is an example of diffusion. The particles of the perfume move freely in the air and spread throughout the room due to the motion of particles.
Q4. What conclusion can be drawn if water level does not rise after dissolving salt in it?
A) Salt disappears completely
B) Water particles become larger
C) Salt particles occupy spaces between water particles
D) Water gets converted to saltwater
✅ Correct Answer: C) Salt particles occupy spaces between water particles
Explanation:
The salt particles fit into the spaces between water molecules, indicating that there are gaps between particles of matter.
Q5. Repeated dilution of potassium permanganate shows which property of particles?
A) Particles are visible
B) Particles change shape
C) Particles have mass
D) Particles are very small and present in large numbers
✅ Correct Answer: D) Particles are very small and present in large numbers
Explanation:
Even after multiple dilutions, the water remains colored. This proves that a few crystals contain millions of tiny particles, which are extremely small.
Q6. Which of the following proves that matter is not continuous but made up of particles?
A) Water boils on heating
B) Ice floats on water
C) Smell of incense stick spreads across the room
D) Mercury is a liquid metal
✅ Correct Answer: C) Smell of incense stick spreads across the room
Explanation:
This diffusion of smell shows that particles are in constant motion and spread through air, confirming the particulate nature of matter.
Q7. What happens when Dettol is added to water and diluted repeatedly?
A) Smell increases
B) Smell disappears completely
C) Smell remains detectable even in small quantity
D) Dettol forms a layer on water
✅ Correct Answer: C) Smell remains detectable even in small quantity
Explanation:
The strong smell of Dettol is due to tiny particles spreading in water and air, proving the particles are very small and diffusive.
Q8. The ability of particles to occupy the spaces between other particles is demonstrated in:
A) Evaporation
B) Dissolution of salt in water
C) Boiling of water
D) Freezing of ice
✅ Correct Answer: B) Dissolution of salt in water
Explanation:
Salt particles fit in between water particles, showing the existence of inter-particle space and small size of particles.
Q9. Which activity shows that particles are in motion and constantly move?
A) Ice melting
B) Mixing of blue ink in water
C) Boiling of alcohol
D) Condensation of steam
✅ Correct Answer: B) Mixing of blue ink in water
Explanation:
The ink spreads evenly in water without stirring, proving particles move continuously, a phenomenon called diffusion.
Q10. Why can a small amount of a substance affect a large volume of another substance?
A) Due to chemical reaction
B) Due to external force
C) Because matter is heavy
D) Because particles are very small and numerous
✅ Correct Answer: D) Because particles are very small and numerous
Explanation:
Even a small quantity contains millions of particles, which can spread throughout a large volume, showing how effective and tiny these particles are.
Q11. Which of the following is the best example to demonstrate diffusion in gases?
A) Dissolving salt in water
B) Spreading of ink in water
C) Smell of food reaching you from the kitchen
D) Mixing sugar in tea
✅ Correct Answer: C) Smell of food reaching you from the kitchen
Explanation:
This shows that gas particles move freely and spread rapidly. This is diffusion in gases – a direct result of the motion and small size of gas particles.
Q12. What does the uniform mixing of ink in water without stirring demonstrate?
A) Ink is heavier than water
B) Ink and water react chemically
C) Particles of matter move continuously
D) Ink particles float on water
✅ Correct Answer: C) Particles of matter move continuously
Explanation:
The movement of ink particles without stirring proves that particles of matter are in constant motion, leading to uniform mixing.
Q13. Which one of the following is not true about particles of matter?
A) They have space between them
B) They are always stationary
C) They are very small
D) They attract each other
✅ Correct Answer: B) They are always stationary
Explanation:
Particles of matter are never stationary. They are in a state of continuous motion, which causes processes like diffusion and mixing.
Q14. The property that explains how gases spread quickly is:
A) Compressibility
B) Density
C) Diffusibility
D) Shape
✅ Correct Answer: C) Diffusibility
Explanation:
Diffusion is the process by which particles move from higher concentration to lower concentration and mix with each other. Gases diffuse the fastest because their particles are far apart and move quickly.
Q15. When potassium permanganate is added to water, why does the purple color spread?
A) It reacts with water
B) It forms a suspension
C) Its particles dissolve and diffuse
D) It releases dye in water
✅ Correct Answer: C) Its particles dissolve and diffuse
Explanation:
The color spreads due to diffusion of particles in water. This shows that particles are constantly moving and can mix spontaneously.
Q16. Which statement correctly explains that particles are very small in size?
A) Sugar can be crushed easily
B) Salt dissolves in water
C) A crystal of KMnO₄ colors 1000 L of water
D) Dettol smells strong even in closed bottles
✅ Correct Answer: C) A crystal of KMnO₄ colors 1000 L of water
Explanation:
A very small quantity coloring a huge amount of water proves that particles are extremely tiny and numerous.
Q17. What does the dissolution of sugar in water without increasing volume tell us?
A) Sugar melts in water
B) Sugar evaporates
C) There are spaces between water particles
D) Water compresses the sugar
✅ Correct Answer: C) There are spaces between water particles
Explanation:
The fact that sugar disappears without changing volume indicates that its particles fit into the spaces between water particles.
Q18. Which characteristic of particles allows salt to dissolve in water?
A) They are large
B) They are static
C) They have strong bonds
D) They have spaces between them
✅ Correct Answer: D) They have spaces between them
Explanation:
The inter-particle spaces in water allow salt particles to slip in, leading to dissolution without a rise in water level.
Q19. Which of these proves the presence of motion in particles of matter?
A) Change in state
B) Evaporation
C) Diffusion of bromine vapors
D) Melting of ice
✅ Correct Answer: C) Diffusion of bromine vapors
Explanation:
Bromine vapors spread throughout the air in a container without shaking or wind, proving constant particle motion.
Q20. What will happen if we keep on diluting potassium permanganate solution repeatedly?
A) Color will become lighter but never disappear
B) It will become darker
C) KMnO₄ will settle down
D) It will form a layer on top
✅ Correct Answer: A) Color will become lighter but never disappear
Explanation:
Even after multiple dilutions, a faint color remains due to extremely small and abundant particles still being present in the solution.
📘 Assertion and Reason Questions: Physical Nature of Matter
Q1.
Assertion (A): Matter is made up of very small particles.
Reason (R): A small crystal of potassium permanganate can color a large volume of water.
Options:
A) Both A and R are true and R is the correct explanation of A.
B) Both A and R are true but R is not the correct explanation of A.
C) A is true but R is false.
D) A is false but R is true.
✅ Correct Answer: A) Both A and R are true and R is the correct explanation of A.
Explanation:
Potassium permanganate, even in a tiny quantity, spreads its color in large amounts of water. This proves that matter is made of very small particles, and the reason directly supports the assertion.
Q2.
Assertion (A): The smell of perfume spreads throughout the room quickly.
Reason (R): Gas particles have large spaces between them and move freely.
Options:
A) Both A and R are true and R is the correct explanation of A.
B) Both A and R are true but R is not the correct explanation of A.
C) A is true but R is false.
D) A is false but R is true.
✅ Correct Answer: A) Both A and R are true and R is the correct explanation of A.
Explanation:
The free movement and large spacing between gas particles allow them to diffuse rapidly, making the perfume smell spread across the room. Thus, both the assertion and the reason are correct, and R explains A.
Q3.
Assertion (A): When sugar dissolves in water, the level of water increases.
Reason (R): Sugar particles occupy space in between water molecules.
Options:
A) Both A and R are true and R is the correct explanation of A.
B) Both A and R are true but R is not the correct explanation of A.
C) A is false but R is true.
D) Both A and R are false.
✅ Correct Answer: C) A is false but R is true.
Explanation:
Water level does not increase when sugar is added because sugar particles fit into the spaces between water particles. Hence, the assertion is false, but the reason is true.
Q4.
Assertion (A): Particles of matter are at rest.
Reason (R): Diffusion does not occur in solids.
Options:
A) Both A and R are true and R is the correct explanation of A.
B) Both A and R are true but R is not the correct explanation of A.
C) A is true but R is false.
D) Both A and R are false.
✅ Correct Answer: D) Both A and R are false.
Explanation:
Particles of matter are not at rest; they are in constant motion. Also, diffusion can occur even in solids (though very slowly, like when two metal blocks are pressed together over time). So both statements are incorrect.
Q5.
Assertion (A): A small amount of Dettol retains its smell even after repeated dilution.
Reason (R): Dettol is insoluble in water.
Options:
A) Both A and R are true and R is the correct explanation of A.
B) Both A and R are true but R is not the correct explanation of A.
C) A is true but R is false.
D) A is false but R is true.
✅ Correct Answer: C) A is true but R is false.
Explanation:
Dettol is soluble in water, and it retains its smell after dilution because its particles are tiny, numerous, and spread throughout. So the assertion is true, but the reason is incorrect.
Q6.
Assertion (A): When two gases are mixed, they combine rapidly.
Reason (R): Gases have high kinetic energy and large intermolecular space.
Options:
A) Both A and R are true and R is the correct explanation of A.
B) Both A and R are true but R is not the correct explanation of A.
C) A is true but R is false.
D) A is false but R is true.
✅ Correct Answer: A) Both A and R are true and R is the correct explanation of A.
Explanation:
Because gas particles are in constant, high-speed motion and have large gaps between them, they diffuse rapidly into one another. Hence, both the assertion and reason are true, and R explains A.
Q7.
Assertion (A): A substance disappears when it dissolves in water.
Reason (R): Dissolving breaks particles into atoms.
Options:
A) Both A and R are true and R is the correct explanation of A.
B) Both A and R are true but R is not the correct explanation of A.
C) A is true but R is false.
D) A is false but R is true.
✅ Correct Answer: C) A is true but R is false.
Explanation:
The substance disappears because it breaks into very small particles and spreads uniformly in water, not into atoms. Therefore, A is true, but R is false.
Q8.
Assertion (A): Potassium permanganate solution becomes colorless after 10 dilutions.
Reason (R): The particles of potassium permanganate are destroyed on dilution.
Options:
A) Both A and R are true and R is the correct explanation of A.
B) Both A and R are true but R is not the correct explanation of A.
C) A is false but R is true.
D) Both A and R are false.
✅ Correct Answer: D) Both A and R are false.
Explanation:
Even after multiple dilutions, the solution remains faintly colored. The particles are not destroyed, they just become fewer per volume. So both statements are false.
🏠 Case Study-Based MCQs: Physical Nature of Matter in Daily Life
✍️ Case Study 1: Smell of Cookies & Sugar in Water
Riya was sitting in her living room when she noticed the smell of freshly baked cookies from the kitchen, even though the door was closed. Curious, she decided to perform a small experiment. She added a spoonful of sugar into a glass of water and noticed that the sugar disappeared without stirring and the water level did not rise. Later, she added a few drops of Dettol in another glass of water and observed that the smell could be detected even after several rounds of dilution.
📌 Based on the above case study, answer the following questions:
Q1. Why could Riya smell the cookies from the kitchen even with the door closed?
A) Smell molecules are heavier and settle down
B) Gas particles are larger and move slowly
C) Gas particles move freely and diffuse in air
D) Solid particles from cookies move in the air
✅ Correct Answer: C) Gas particles move freely and diffuse in air
Explanation:
The smell of cookies spreads due to diffusion of gas particles. Gases have large spaces between particles and move quickly, allowing smells to travel even through closed spaces.
Q2. What does the disappearance of sugar in water without a rise in level suggest?
A) Sugar melts in water
B) Sugar reacts with water
C) Sugar particles fill spaces between water particles
D) Sugar particles sink to the bottom
✅ Correct Answer: C) Sugar particles fill spaces between water particles
Explanation:
This demonstrates that matter has spaces between particles. The sugar particles fit into these spaces and dissolve without increasing the water level.
Q3. The smell of Dettol was observed even after several dilutions. What conclusion can be drawn from this observation?
A) Dettol is volatile and evaporates easily
B) Dettol particles are chemically reactive
C) Dettol particles are very large
D) Dettol particles are destroyed in water
✅ Correct Answer: A) Dettol is volatile and evaporates easily
Explanation:
Volatile substances like Dettol release vapors, which diffuse in air. The fact that smell is still detected after dilution shows particles are small and in constant motion.
Q4. Which property of matter is not demonstrated in Riya’s observations?
A) Matter is made up of particles
B) Particles of matter are very large
C) Particles of matter are in constant motion
D) There is space between particles
✅ Correct Answer: B) Particles of matter are very large
Explanation:
Riya’s activities show that particles are very small, as just a small amount of substance can spread or disappear. Hence, B is not correct.
Q5. Which scientific concept explains why Riya did not need to stir the sugar to make it disappear?
A) Solubility of liquids
B) Reaction of sugar with water
C) Random motion of particles
D) Change in state of matter
✅ Correct Answer: C) Random motion of particles
Explanation:
Particles of matter are in constant, random motion. This causes dissolution of sugar even without stirring, demonstrating movement of particles.
🧪 Case Study 2: The Ink Drop Experiment
Aryan was performing an experiment at home. He took a transparent glass and filled it with water. Then he carefully added a drop of blue ink without stirring. After a few minutes, he noticed that the entire glass of water turned light blue, even though he hadn’t touched or shaken the glass. He was amazed that a single drop could spread evenly without any external force.
📌 MCQs Based on Case Study 2
Q1. What property of matter does Aryan’s observation demonstrate?
A) Matter is continuous
B) Matter cannot change color
C) Particles of matter are at rest
D) Particles of matter are in constant motion
✅ Correct Answer: D) Particles of matter are in constant motion
Explanation:
The spreading of ink throughout the water without stirring shows that particles are always moving, which causes diffusion.
Q2. What would happen if Aryan used hot water instead of cold water?
A) The ink would settle at the bottom
B) Ink would diffuse more slowly
C) Ink would not diffuse at all
D) Ink would diffuse faster
✅ Correct Answer: D) Ink would diffuse faster
Explanation:
In hot water, particles move faster due to higher kinetic energy, so diffusion occurs more rapidly.
Q3. Why did Aryan observe a uniform color after a few minutes?
A) Ink reacted with water
B) Ink particles became larger
C) Ink particles diffused in water
D) Water absorbed the ink molecules
✅ Correct Answer: C) Ink particles diffused in water
Explanation:
Diffusion caused the ink particles to spread evenly through water, resulting in a uniform color.
🏥 Case Study 3: Hospital Visit and Smell of Spirit
Priya went to a hospital with her mother. As soon as she entered, she noticed the strong smell of antiseptic spirit even though no bottle was open nearby. She asked the nurse why she could smell it. The nurse explained that spirit is a volatile liquid and evaporates easily, allowing its particles to mix with air and travel to the nose.
📌 MCQs Based on Case Study 3
Q1. Why could Priya smell the spirit without touching or seeing the bottle?
A) Spirit is heavier than air
B) Spirit reacts with air to form a gas
C) Spirit vapors dissolve in air and diffuse
D) Spirit turns into solid on evaporation
✅ Correct Answer: C) Spirit vapors dissolve in air and diffuse
Explanation:
Spirit is volatile and forms vapors that diffuse quickly in air, allowing us to smell it even from a distance.
Q2. What does the strong smell of spirit show about the nature of matter?
A) Particles are rigid
B) Particles are large
C) Particles are in motion and have space between them
D) Particles do not move
✅ Correct Answer: C) Particles are in motion and have space between them
Explanation:
Smell spreading in air is possible because gas particles are constantly moving and have large gaps between them.
Q3. Which process allows the antiseptic smell to reach Priya’s nose?
A) Fusion
B) Diffusion
C) Condensation
D) Compression
✅ Correct Answer: B) Diffusion
Explanation:
Diffusion is the process by which gas or vapor particles spread in air from a region of high concentration to low concentration.
🧪 Quiz: Physical Nature of Matter
✅ Quick Revision Points :
- Matter is not continuous but made up of tiny particles.
- These particles have space between them.
- Sugar and salt dissolve because their particles fill the spaces between water molecules.
- Potassium permanganate and Dettol show that particles are very small and easily spread.
- The particulate nature of matter explains phenomena like diffusion, solubility, and mixing.
💡 Do You Know?
- Just 2 crystals of potassium permanganate can colour 1000 liters of water faintly!
- Smell of Dettol or perfume spreads in a room due to diffusion—a property of tiny moving particles.



